Physics of Uranium and Nuclear Energy
- Nuclear reactors work by containing and controlling the physical process of nuclear fission.
- Radioactive decay of both fission products and transuranic elements formed in a reactor yield heat even after fission has ceased.
- Fission reactions may be moderated to increase fission, or unmoderated to breed further fuel.
- For reactors using light water as moderator, enriched uranium is required.
- Isotope separation to achieve uranium enrichment is by physical processes.
Neutrons
Neutrons in motion are the starting point for everything that happens in a nuclear reactor. For more information on how a nuclear power plant works, see information page Nuclear Power Reactors.
When a neutron passes near to a heavy nucleus, for example uranium-235 (U-235), the neutron may be captured by the nucleus and this may or may not be followed by fission. Capture involves the addition of the neutron to the uranium nucleus to form a new compound nucleus. A simple example is U-238 + n ==> U-239, which represents formation of the nucleus U-239. The new nucleus may decay into a different nuclide. In this example, U-239 becomes Np-239 after emission of a beta particle (electron). But in certain cases the initial capture is rapidly followed by the fission of the new nucleus. Whether fission takes place, and indeed whether capture occurs at all, depends on the velocity of the passing neutron and on the particular heavy nucleus involved.
Nuclear fission
Fission may take place in any of the heavy nuclei after capture of a neutron. However, low-energy (slow, or thermal) neutrons are able to cause fission only in those isotopes of uranium and plutonium whose nuclei contain odd numbers of neutrons (e.g. U-233, U-235, and Pu-239). Thermal fission may also occur in some other transuranic elements whose nuclei contain odd numbers of neutrons. For nuclei containing an even number of neutrons, fission can only occur if the incident neutrons have energy above about one million electron volts (MeV). (Newly-created fission neutrons are in this category and move at about 7% of the speed of light, while moderated neutrons move a lot slower, at about eight times the speed of sound).
Neutron cross-sections for fission of uranium and plutonium
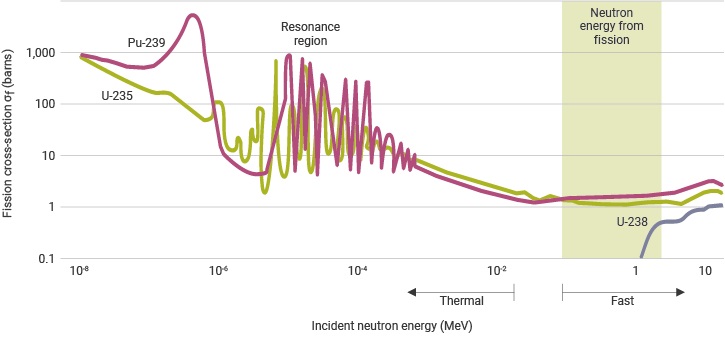
Notes: both scales are logarithmic. 1 barn = 10-28 m2, 1 MeV = 1.6 x 10-13 J
Source: NEA, Plutonium fuel – an assessment (1989); Taube, Plutonium – a general survey (1974)
A neutron is said to have thermal energy when it has slowed down to be in thermal equilibrium with the surroundings (when the kinetic energy of the neutrons is similar to that possessed by the surrounding atoms due to their random thermal motion). Hence the main application of uranium fission today is in thermal reactors fuelled by U-235 and incorporating a moderator such as water to slow the neutrons down. The most common examples of this are light water reactors*.
* There are two main varieties, pressurized water reactors and boiling water reactors. For more information see page on Nuclear Power Reactors.
Other heavy nuclei that are fissile (implying thermal fission) are U-233, Pu-239 and Pu-241. Each of these is produced artificially in a nuclear reactor, from the fertile nuclei Th-232 (in certain reactors), U-238 and Pu-240 respectively. U-235 is the only naturally occurring isotope which is thermally fissile, and it is present in natural uranium at a concentration of 0.7%. U-238 and Th-232 are the main naturally-occurring fertile isotopes.
The probability that fission or any another neutron-induced reaction will occur is described by the neutron cross-section for that reaction. This may be imagined as an area surrounding the target nucleus and within which the incoming neutron must pass if the reaction is to take place. The fission and other cross-sections increase greatly as the neutron velocity reduces from around 20,000 km/s to 2 km/s, making the likelihood of some interaction greater. In nuclei with an odd number of neutrons, such as U-235, the fission cross-section becomes very large at the thermal energies of slow neutrons.
As implied previously, high-energy (> 0.1 MeV) neutrons are travelling too quickly to have much interaction with the nuclei in the fuel. We therefore say that the fission cross-section of those nuclei is much reduced at high neutron energies relative to its value at thermal energies (for slow neutrons). It is nonetheless possible to use this so-called fast fission in a fast neutron reactor whose design minimises the moderation of the high-energy neutrons produced in the fission process. See below.
Nuclear fission – the process
Using U-235 in a thermal reactor as an example, when a neutron* is captured the total energy is distributed amongst the 236 nucleons (protons & neutrons) now present in the compound nucleus. This nucleus is relatively unstable, and it is likely to break into two fragments of around half the mass. These fragments are nuclei found around the middle of the Periodic Table and the probabilistic nature of the break-up leads to several hundred possible combinations. Creation of the fission fragments is followed almost instantaneously by emission of a number of neutrons (typically 2 or 3, average 2.45), which enable the chain reaction to be sustained.
* The chain reaction is started by inserting some beryllium mixed with polonium, radium or other alpha-emitter. Alpha particles from the decay cause a release of neutrons from the beryllium as it turns to carbon-12.
Distribution of fission products
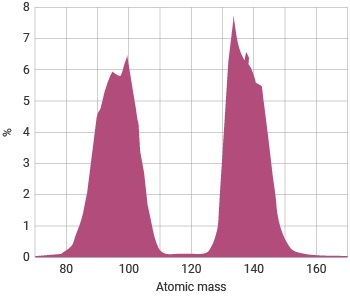
Notes: from fuel 65% U, 35% Pu fissions
Source: World Nuclear Association
About 85% of the energy released is initially the kinetic energy of the fission fragments. However, in solid fuel they can only travel a microscopic distance, so their energy becomes converted into heat. The balance of the energy comes from gamma rays emitted during or immediately following the fission process and from the kinetic energy of the neutrons. Some of the latter are immediate (so-called prompt neutrons), but a small proportion (0.66% for U-235, 0.27% for U-233, 0.23% for Pu-239) is delayed, as these are associated with the radioactive decay of certain fission products. The longest delayed neutron group has a half-life of about 56 seconds.
The delayed neutron release is the crucial factor enabling a chain reacting system (or reactor) to be controllable and to be able to be held precisely critical. At criticality the chain reacting system is exactly in balance, such that the number of neutrons produced in fissions remains constant. This number of neutrons may be completely accounted for by the sum of those causing further fissions, those otherwise absorbed, and those leaking out of the system. Under these circumstances the power generated by the system remains constant. To raise or lower the power, the balance must be changed (using the control system) so that the number of neutrons present (and hence the rate of power generation) is either reduced or increased. The control system is used to restore the balance when the desired new power level is attained.
The number of neutrons and the specific fission products from any fission event are governed by statistical probability, in that the precise break up of a single nucleus cannot be predicted. However, conservation laws require the total number of nucleons and the total energy to be conserved. The fission reaction in U-235 produces fission products such as Ba, Kr, Sr, Cs, I and Xe with atomic masses distributed around 95 and 135. Examples may be given of typical reaction products, such as:
U-235 + n ===> Ba-144 + Kr-90 + 2n + about 200 MeV
U-235 + n ===> Ba-141 + Kr-92 + 3n + 170 MeV
U-235 + n ===> Zr-94 + Te-139 + 3n + 197 MeV
In such an equation, the number of nucleons (protons + neutrons) is conserved, e.g. 235 + 1 = 141 + 92 + 3, but a small loss in atomic mass may be shown to be equivalent to the energy released. Both the barium and krypton isotopes subsequently decay and form more stable isotopes of neodymium and yttrium, with the emission of several electrons from the nucleus (beta decays). It is the beta decays, with some associated gamma rays, which make the fission products highly radioactive. This radioactivity (by definition!) decreases with time.
The total binding energy released in fission of an atomic nucleus varies with the precise break up, but averages about 200 MeV* for U-235 or 3.2 x 10-11 joule. This is about 82 TJ/kg. That from U-233 is about the same, and that from Pu-239 is about 210 MeV* per fission. (This contrasts with 4 eV or 6.5 x 10-19 J per atom of carbon burned in fossil fuels.)
* these are total available energy release figures, consisting of kinetic energy values (Ek) of the fission fragments plus neutron, gamma and delayed energy releases which add about 30 MeV.
About 6% of the heat generated in the reactor core originates from radioactive decay of fission products and transuranic elements formed by neutron capture, mostly the former. This must be allowed for when the reactor is shut down, since heat generation continues after fission stops. It is this decay which makes used fuel initially generate heat and hence need cooling, as very publicly demonstrated in the Fukushima accident when cooling was lost an hour after shutdown and the fuel was still producing about 1.5% of its full-power heat. Even after one year, typical used fuel generates about 10 kW of decay heat per tonne, decreasing to about 1 kW/t after ten years.
Neutron Capture: Transuranic elements & activation products
Neutrons may be captured by non-fissile nuclei, and some energy is produced by this mechanism in the form of gamma rays as the compound nucleus de-excites. The resultant new nucleus may become more stable by emitting alpha or beta particles. Neutron capture by one of the uranium isotopes will form what are called transuranic elements, actinides beyond uranium in the periodic table.
Since U-238 is the major proportion of the fuel element material in a thermal reactor, capture of neutrons by U-238 and the creation of U-239 is an important process.
- U-239 quickly emits a beta particle to become neptunium-239.
- Np-239 in turn emits a beta particle to become plutonium-239, which is relatively stable.
- Some Pu-239 nuclei may capture a neutron to become Pu-240, which is less stable.
- By further neutron capture, some Pu-240 nuclei may in turn form Pu-241.
- Pu-241 also undergoes beta decay to americium-241 (the heart of household smoke detectors).
As already noted, Pu-239 is fissile in the same way as U-235, i.e. with thermal neutrons. It is the other main source of energy in any nuclear reactor. If fuel is left in the reactor for a typical three years, about two-thirds of the Pu-239 is fissioned with the U-235, and it typically contributes about one-third of the energy output. The masses of its fission products are distributed around 100 and 135 atomic mass units. One difference is that Pu-239 fission in a thermal reactor results in 2.9 neutrons on average, instead of almost 2.5 for U-235, and its fission cross-section is three times its capture cross-section so that about one-quarter of reactions result in the formation of Pu-240 which is not fissile. In a fast reactor, Pu-239 produces more neutrons per fission (e.g. at 2 MeV: four), so is better suited to the fast neutron spectrum (see below).
The main transuranic constituents of used fuel are isotopes of plutonium, curium, neptunium and americium, the last three being 'minor actinides'. These are alpha-emitters and have long half-lives, decaying on a similar time scale to the uranium isotopes. They are the reason that used fuel needs secure disposal beyond the few thousand years or so which might be necessary for the decay of fission products alone.
Activity of high-level waste from one tonne of used fuel
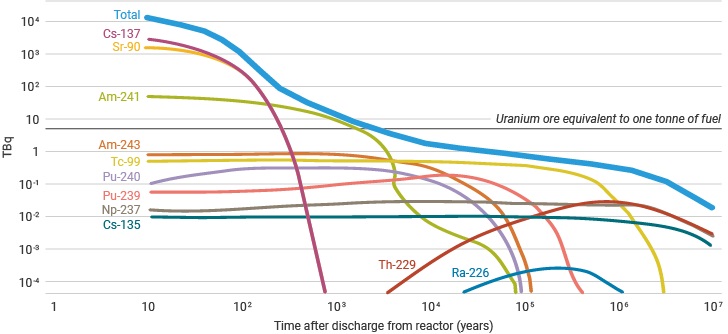
Source: IAEA, 1992, Radioactive waste management
Apart from transuranic elements in the reactor fuel, activation products are formed wherever neutrons impact on any other material surrounding the fuel. Activation products in a reactor (and particularly its steel components exposed to neutrons) range from tritium (H-3) and carbon-14, to cobalt-60, iron-55 and nickel-63. The latter four radioisotopes create difficulties during eventual demolition of the reactor, and affect the extent to which materials can be recycled.
Fast Neutron Reactors
In a fast neutron reactor the fuel in the core is Pu-239 and the abundant neutrons which leak from the core breed more Pu-239 in a fertile blanket of U-238 around the core. A minor fraction of U-238 might be subject to fission, but most of the neutrons reaching the U-238 blanket will have lost some of their original energy and are therefore subject only to capture and thus breeding of Pu-239. Cooling of the fast reactor core requires a heat transfer medium which has minimal moderation of the neutrons, and hence liquid metals are used, typically sodium.
Such reactors can be up to 100 times more efficient at converting fertile material than ordinary thermal reactors because of the arrangement of fissile and fertile materials, and there is some advantage from the fact that Pu-239 yields more neutrons per fission than U-235. Although both yield more neutrons per fission when split by fast rather than slow neutrons, this is incidental since the fission cross sections are much smaller at high neutron energies. While the conversion ratio (the ratio of new fissile nuclei to fissioned nuclei) in a normal reactor is around 0.6, that in a fast reactor may exceed 1.0. Fast neutron reactors may be designed as breeders to yield more fissile material than they consume, or to be plutonium burners to dispose of excess plutonium. A plutonium burner would be designed without a breeding blanket, simply with a core optimised for plutonium fuel, and this is the likely shape of future fast neutron reactors, even if they have some breeding function.
For instance, the Fast Breeder Reactor was originally conceived to extend the world's uranium resources, and could do this by a factor of about 60. Although several countries ran extensive fast breeder reactor development programs, major technical and materials problems were encountered. To the extent that these programs permitted, it was not established that any of the designs would have been commercially competitive with existing light water reactors. An important aspect of fast reactor economics lies in the value of the plutonium fuel which is bred; unless this shows an advantage relative to contemporary costs for uranium, there would be little benefit from the use of this type of reactor. This point was driven home in the 1980s and 1990s by recognition of the abundance of uranium in geological resources and its relatively low price then.
Fast reactors have a strong negative temperature coefficient (the reaction slows as the temperature rises unduly), an inherent safety feature, and the basis of automatic load-following in some new designs, by controlling the coolant flow.
Today there is renewed interest in fast neutron reactors for three reasons. First is their potential roles in burning long-lived actinides recovered from light water reactor used fuel, secondly a short-term role in the disposal of ex-military plutonium, and thirdly enabling much fuller use of the world's uranium resources (even though these re abundant). In all respects the technology is important to long-term considerations of world energy sustainability.
For more information, see page on Fast Neutron Reactors.
Control of Fission
Fission of U-235 nuclei typically releases 2 or 3 neutrons, with an average of almost 2.5. One of these neutrons is needed to sustain the chain reaction at a steady level of controlled criticality; on average, the others leak from the core region or are absorbed in non-fission reactions. Neutron-absorbing control rods are used to adjust the power output of a reactor. These typically use boron and/or cadmium (both are strong neutron absorbers) and are inserted among the fuel assemblies. When they are slightly withdrawn from their position at criticality, the number of neutrons available for ongoing fission exceeds unity (i.e. criticality is exceeded) and the power level increases. When the power reaches the desired level, the control rods are returned to the critical position and the power stabilises.
The ability to control the chain reaction is entirely due to the presence of the small proportion of delayed neutrons arising from fission (0.66% for U-235, 0.27% for U-233, 0.23% for Pu-239). Without these, any change in the critical balance of the chain reaction would lead to a virtually instantaneous and uncontrollable rise or fall in the neutron population. It is also relevant to note that safe design and operation of a reactor sets very strict limits on the extent to which departures from criticality are permitted. These limits are built in to the overall design.
While fuel is being burned in the reactor, it is gradually accumulating fission products and transuranic elements which cause additional neutron absorption. The control system has to be adjusted to compensate for the increased absorption. When the fuel has been in the reactor for three years or so, this build-up in absorption, along with the metallurgical changes induced by the constant neutron bombardment of the fuel materials, dictates that the fuel should be replaced. This effectively limits the burn-up to about half of the fissile material, and the fuel assemblies must then be removed and replaced with fresh fuel. Fuel life can be extended by use of burnable poisons such as gadolinium, the effect of which compensates for the build-up of neutron absorbers.
Neutrons released in fission are initially fast (velocity about 109 cm/sec, or energy above 1 MeV), but fission in U-235 is most readily caused by slow neutrons (velocity about 105 cm/s, or energy about 0.02 eV). A moderator material comprising light atoms thus surrounds the fuel rods in a reactor. Without absorbing too many, it must slow down the neutrons in elastic collisions (compare it with collisions between billiard balls on an atomic scale). In a reactor using natural (unenriched) uranium the only suitable moderators are graphite and heavy water (these have low levels of unwanted neutron absorption). With enriched uranium (i.e. increased concentration of U-235), ordinary (light) water may be used as moderator. (Water is also commonly used as a coolant, to remove the heat and generate steam.)
Other features may be used in different reactor types to control the chain reaction. For instance, a small amount of boron may be added to the cooling water and its concentration reduced progressively as other neutron absorbers build up in the fuel elements. (For emergency situations, provision may be made for rapidly adding an excessive quantity of boron to the water.)
Commercial power reactors are usually designed to have negative temperature and void coefficients. The significance of this is that if the temperature should rise beyond its normal operating level, or if boiling should occur beyond an acceptable level, the balance of the chain reaction is affected so as to reduce the rate of fission and hence reduce the temperature. One mechanism involved is the Doppler effect, whereby U-238 absorbs more neutrons as the temperature rises, thereby pushing the neutron balance towards subcritical. Another important mechanism, in light water reactors, is that the formation of steam within the water moderator will reduce its density and hence its moderating effect, and this again will tilt the neutron balance towards subcritical.
In naval reactors used for propulsion, where fuel changes are inconvenient, the fuel is enriched to higher levels initially and burnable poisons – neutron absorbers – are incorporated and the initial fuel load may last the life of the vessel. Hence as the fission products and transuranic elements accumulate, the 'poison' is depleted and the two effects tend to cancel one another out. To maximise the burn-up of commercial reactor fuel, burnable poisons such as gadolinium are increasingly used, along with increasing enrichment towards 5% U-235. Gadolinium is incorporated in the ceramic fuel pellets. An alternative is zirconium bromide integral fuel burnable absorber (IFBA) as a thin coating on normal pellets. It is now used in most US reactors and a few in Asia.
While fuel is being burned in the reactor, it is gradually accumulating fission products and transuranic elements which cause additional neutron absorption. The control system has to be adjusted to compensate for the increased absorption. When the fuel has been in the reactor for three years or so, this build-up in absorption, along with the metallurgical changes induced by the constant neutron bombardment of the fuel materials, dictates that the fuel should be replaced. This effectively limits the burn-up to about half of the fissile material, and the fuel assemblies must then be removed and replaced with fresh fuel. Fuel life can be extended by use of burnable poisons such as gadolinium, the effect of which compensates for the build-up of neutron absorbers.
Fission in uranium exploration
Traditionally, most uranium exploration has used gamma measurement from the uranium orebody. However, this comes from decay products, not uranium itself. Where the uranium has been leached from the original orebody with its decay products and deposited elsewhere, in buried river channels for instance, gamma measurements do not give a good indication of uranium concentrations. The best indication is from causing a little fission.
A portable prompt fission neutron (PFN) logging tool employs a neutron source and a neutron detector. The neutron source irradiates the uranium deposit and prompt or delayed neutrons resulting from fission of any uranium present in the formation are detected and recorded. This is the only reliable way of geophysical measurement of some uranium deposits.
Nuclear fusion
While not strictly from uranium, a great deal of research is being undertaken to harness nuclear fusion power. A number of reactions are possible, but the one which is within reach technologically is the deuterium-tritium reaction. This has proven possible in a small reactor – the Joint European Torus (JET) – where 16 MW was achieved briefly, and 5 MW was sustained in 1997. This work is now being scaled up internationally with ITER, being built in France.
The reaction is:
H-2 + H-3 ===> He-4 + neutron + 17.6 MeV
Tritium can be bred from lithium-6 in a blanket around the torus, using neutrons from the reaction:
Li-6 + neutron ==⇒ He-4 + H-3 (tritium) + 4.8 MeV
Deuterium is relatively abundant in seawater.
For more information, see page on Nuclear Fusion.
Uranium enrichment
Enrichment, or isotope separation, is a physical process to concentrate (‘enrich’) one isotope relative to others. The most common types of commercial power reactor use water for both moderator and coolant. Criticality may only be achieved with a water moderator if the fuel is enriched. Enrichment increases the proportion of the fissile isotope U-235 about five- to sevenfold from the 0.7% of U-235 found in natural uranium. Enrichment usually relies on the small mass difference between atoms of the two isotopes U-238 and U-235. The two main enrichment (or isotope separation) processes are diffusion (gas diffusing under pressure through a membrane containing microscopic pores) and centrifugation.
For more information, see page on Uranium Enrichment.
Notes & references
General sources
Alan Marks
ANSTO
Albert Reynolds, 1996, Bluebells and Nuclear Energy, Cogito Press
Anthony Nero jr, 1979, A Guidebook to Nuclear Energy, UC Press
C.R.Hill & R.S.Pease, 1999, Nuclear Electricity – an aide memoir, in Nuclear Energy, Promise or Peril? World Scientific