Naturally-Occurring Radioactive Materials (NORM)
- Radioactive materials which occur naturally and where human activities increase the exposure of people to ionising radiation are known by the acronym 'NORM'.
- NORM results from activities such as burning coal, making and using fertilisers, oil and gas production.
- Uranium mining exposes those involved to NORM in the uranium orebody.
- Radon in homes is one occurrence of NORM which may give rise to concern and action to control it, by ventilation.
All minerals and raw materials contain radionuclides of natural origin. The most important for the purposes of radiation protection are the radionuclides in the U-238 and Th-232 decay series. For most human activities involving minerals and raw materials, the levels of exposure to these radionuclides are not significantly greater than normal background levels and are not of concern for radiation protection. However, certain work activities can give rise to significantly enhanced exposures that may need to be controlled by regulation. Material giving rise to these enhanced exposures has become known as naturally occurring radioactive material (NORM).
NORM potentially includes all radioactive elements found in the environment. However, the term is used more specifically for all naturally occurring radioactive materials where human activities have increased the potential for exposure compared with the unaltered situation. Concentrations of actual radionuclides may or may not have been increased; if they have, the term technologically-enhanced NORM (TENORM) may be used.
Long-lived radioactive elements such as uranium, thorium and potassium and any of their decay products, such as radium and radon are examples of NORM. These elements have always been present in the Earth's crust and atmosphere, and are concentrated in some places, such as uranium orebodies which may be mined. The term NORM exists also to distinguish ‘natural radioactive material’ from anthropogenic sources of radioactive material, such as those produced by nuclear power and used in nuclear medicine, where incidentally the radioactive properties of a material maybe what make it useful. However from the perspective of radiation doses to people, such a distinction is completely arbitrary.
Exposure to naturally occurring radiation is responsible for the majority of an average person’s yearly radiation dose (see also Nuclear Radiation and Health Effects paper) and is therefore not usually considered of any special health or safety significance. However certain industries handle significant quantities of NORM, which usually ends up in their waste streams, or in the case of uranium mining, the tailings dam. Over time, as potential NORM hazards have been identified, these industries have increasingly become subject to monitoring and regulation. However, there is as yet little consistency in NORM regulations among industries and countries. This means that material which is considered radioactive waste in one context may not be considered so in another. Also, that which may constitute low-level waste in the nuclear industry might go entirely unregulated in another industry (see section below on recycling and NORM).
The acronym TENORM, or technologically enhanced NORM, is often used to refer to those materials where the amount of radioactivity has actually been increased or concentrated as a result of industrial processes. This paper addresses some of these industrial sources, and for simplicity the term NORM will be used throughout.
Excluding uranium mining and all associated fuel cycle activities, industries known to have NORM issues include:
- The coal industry (mining and combustion)
- The oil and gas industry (production)
- Metal mining and smelting
- Mineral sands (rare earth minerals, titanium and zirconium).
- Fertiliser (phosphate) industry
- Building industry
- Recycling
Another NORM issue relates to radon exposure in homes, particularly those built on granitic ground. Occupational health issues include the exposure of flight crew to higher levels of cosmic radiation, the exposure of tour guides to radon in caves, exposure of miners to radon underground, and exposure of workers in the oil & gas and mineral sands industries to elevated radiation levels in the materials they handle.
NORM sources
The list of isotopes that contribute to natural radiation can be divided into those materials which come from the ground (terrestrial sources – the vast majority) and those which are produced as a result of the interaction of atmospheric gases with cosmic rays (cosmogenic).
NORM levels are typically expressed in one of two ways: Becquerels per kilogram (or gram) indicates level of radioactivity generally or due to a particular isotope, while parts per million (ppm) indicates the concentration of a specific radioisotope in the material.
Terrestrial NORM
Terrestrial NORM consists of radioactive material that comes out of the Earth’s crust and mantle, and where human activity results in increased radiological exposure. The materials may be original (such as uranium and thorium) or decay products thereof, forming part of characteristic decay chain series, or potassium-40. The two most important chains providing nuclides of significance in NORM are the thorium series and the uranium series:
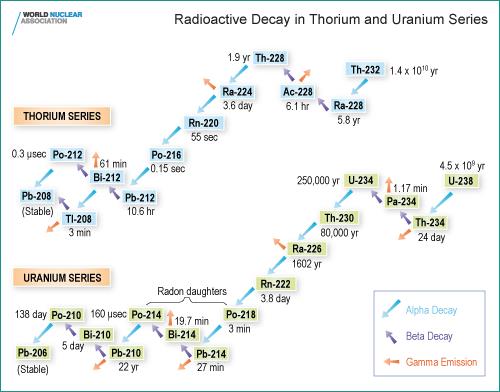
Another major source of terrestrial NORM is potassium 40 (K-40). The long half-life of K-40 (1.25 billion years) means that it still exists in measurable quantities today. It beta decays, mostly to calcium-40, and forms 0.012% of natural potassium which is otherwise made up of stable K-39 and K-41. Potassium is the seventh most abundant element in the Earth’s crust, and K-40 averages 850 Bq/kg there. It is found in many foodstuffs (bananas for example), and indeed fills an important dietary requirement, ending up in our bones. (Humans have about 65 Bq/kg of K-40 and along with those foods are therefore correspondingly radioactive to a small degree. A 70 kg person has 4400 Bq of K-40 – and 3000 Bq of carbon-14.)
Cosmogenic NORM
Cosmogenic NORM is formed as a result of interactions between certain gases in the Earth’s atmosphere and cosmic rays, and is only relevant to this paper due to flying being a common mode of transport. Since most cosmic radiation is deflected by the Earth’s magnetic field or absorbed by the atmosphere, very little reaches the Earth’s surface and cosmogenic radionuclides contribute more to dose at low altitudes than cosmic rays as such. At higher altitudes, the dose due to both increases, meaning that mountain dwellers and frequent flyers are exposed to higher doses than others. For most people, cosmogenic NORM barely contributes to dose – perhaps a few tens of microsieverts per year. By contrast, terrestrial NORM – especially radon – contributes to the majority of natural dose, usually over 1000 microsieverts (1 mSv) per year. Some of the main comsogenic nuclides are shown in Table 1, carbon-14 being important for dating early human activities.
Table 1: Radiological characteristics of cosmogenic NORM
| Nuclide | decay mode | half life |
|---|---|---|
| C-14 | β- | 5700 y |
| H-3 (tritium) | β- | 12.32 y |
| Na-22 | β+ and electron capture | 2.6 y |
| Be-7 | Electron capture | 53.22 d |
NORM and cosmic radiation account for over 85% of an ‘average individual’s’ radiation exposure. Most of the balance is from exposure related to medical procedures. (Exposure from the nuclear fuel cycle - including fallout from the Chernobyl accident - accounts for less than 0.1%.)
Industries producing NORM
Coal Energy – combustion and ash
Over the years there have been many occasions when it was asserted that coal-fired power stations emitted more radioactivity into the environment (from NORM) than was released anywhere in the nuclear fuel cycle. While having some basis in fact, the claim is generally not correct now where deployment of emission reduction technology – scrubbers, filters and flue gas desulphurization – acts to capture solids from this material. More volatile Po-210 and Pb-210 still escape. In China, coal-fired power plants are a major source of radioactivity released to the environment and thus contribute significantly to enhanced NORM there. (Wu et al in NORM VII)
Most coal contains uranium and thorium, as well as their decay products and K-40. The total levels of individual radionuclides typically are not great and are generally about the same as in other rocks near the coal, which varies according to region and geology. Enhanced radionuclide concentration in coal tends to be associated with the presence of other heavy metals and high sulfur content. Table 2 presents some characteristic values,* though coal in some areas can contain notably higher levels than shown. For comparison, the average radioactivity of the Earth’s crust is about 1400 Bq/kg, more than half of it from K-40.
* The first four columns represent four of the 14 nuclides in the uranium decay series, the next two represent two of 10 in the thorium series. (For total activity in any coal, assume these are in serial equilibrium, hence multiply U-238 by 14 and Th-232 by 10, then add K-40.)
Table 2: NORM radionuclide activity in coal (Bq/kg)
| Country | U-238 | Ra-226 | Pb-210 | Po-210 | Th-232 | Ra-228 | K-40 |
|---|---|---|---|---|---|---|---|
| Australia | 8.5-47 | 19-24 | 20-33 | 16-28 | 11-69 | 11-64 | 23-140 |
| Brazil | 72 | 72 | 72 | 62 | 62 | ||
| China | Typical 10-25, up to 5600 | Typical 10-25, up to 29,000 | |||||
| Germany | 10-145, av 32 | 10-63, av 21 | 10-700, av 225 | ||||
| (lignite) | 0-58 | 0-58 | 4-220 | ||||
| Greece (lignite) | 117-390 | 44-206 | 59-205 | 9-41 | |||
| Hungary | 20-480 | 12-97 | 30-384 | ||||
| Poland | Up to 159, av 18 | Up to 123, av 11 | Up to 785 | ||||
| Romania | Up to 415, av 80 | Up to 557, av 126 | Up to 510, av 210 | Up to 580, av 262 | Up to 170, av 62 | ||
| UK | 7-19 | 8-22 | 7-19 | 55-314 | |||
| USA | 6-73 | 8.9-59 | 12-78 | 3-52 | 4-21 | ||
Source: IAEA Technical Reports Series no. 419, Table VII (p 24)
IAEA NORM VII, p8 for China
Dale in ACARP 2006 gives 370 Bq/kg average total for Australian coal
The amounts of radionuclides involved are noteworthy. US, Australian, Indian and UK coals contain up to about 4 ppm uranium, those in Germany up to 13 ppm, and those from Brazil and China range up to 20 ppm uranium. Thorium concentrations are often about three times those of uranium.
During combustion the radionuclides are retained and concentrated in the flyash and bottom ash, with a greater concentration to be found in the flyash. The concentration of uranium and thorium in bottom and flyash can be up to ten times greater than for the burnt coal, while other radionuclides such as Pb-210 and K-40 can concentrate to an even greater degree in the flyash. Some 99% of flyash is typically retained in a modern power station (90% in some older ones). While much flyash is buried in an ash dam, a lot is used in building construction. Table 3 gives some published figures for the radioactivity of ash. There are obvious implications for the use of flyash in concrete.
At a coal-fired power plant in China the amount of polonium-210 aerosol emitted from a coal plant stack was measured and found to be 257 MBq/GW/yr. (Liu et al in NORM VII)
Table 3: NORM radionuclide activity in coal ash and slag (Bq/kg)
| Uranium series, Ra-226 | Thorium series | K-40 | |
|---|---|---|---|
| Hungary | 200-2000 | 20-300 | 300-800 |
| USA | 100-600 | 30-300 | 100-1200 |
| Germany ash | 6-166 | 3-120 | 125-742 |
| Germany slag | 68-245 | 76-170 | 337-1240 |
| Australia | Total: 2630 fly ash 1680, bottom ash 1410 |
||
| Australia: NSW | Total: 3200 | ||
Source: IAEA Technical Reports Series no. 419, p 30; CSIRO for Australia
In 2017 Australia exported 372 million tonnes of coal. With an average of 0.9 ppm uranium and 2.6 ppm thorium, at least 330 tonnes of uranium per year and 970 tonnes of thorium could conceivably be added to published export figures.
In the USA, 858 million tonnes of coal was used in 2013 for electricity production. With an average content of 1.3 ppm uranium and 3.2 ppm thorium, US coal-fired electricity generation in that year gave rise to 1100 tonnes of uranium and 2700 tonnes of thorium in coal ash. In Victoria, Australia, some 65 million tonnes of brown coal is burned annually for electricity production. This contains about 1.6 ppm uranium and 3.0-3.5 ppm thorium, hence about 100 tonnes of uranium and 200 tonnes of thorium is buried in landfill each year in the Latrobe Valley.
It is evident that even at 1 part per million (ppm) U in coal, there is more energy in the contained uranium (if it were to be used in a fast neutron reactor) than in the coal itself. If coal had 25 ppm uranium and that uranium was used simply in a conventional reactor, it would yield half as much thermal energy as the coal.
With increased uranium prices the uranium in ash becomes significant economically. In the 1960s and 1970s, some 1100 tU was recovered from coal ash in the USA. The feasibility depends on grade and the composition of the ash – high acid consumption makes recovery uneconomic.
In 2007, China National Nuclear Corp (CNNC) commissioned Sparton Resources <http://www.spartonres.ca> of Canada with the Beijing No.5 Testing Institute to undertake advanced trials on leaching uranium from coal ash in central Yunnan. In early 2007, Sparton signed an agreement with the Xiaolongtang Guodian Power Company of Yunnan for a program to test and possibly commercialize the extraction of uranium from waste coal ash. Some 250 km southwest of Kunming, the Xiaolongtang, Dalongtang and the Kaiyuan power stations, all located within 20 km of each other burn coal from a centrally located open pit lignite mine with high ash content (20-30%) and very high uranium content. The coal uranium content varies from about 20 to 315 ppm and averages about 65 ppm. The ash averages about 210 ppm U (0.021%U) - above the cut-off level for some uranium mines. The power station ash heap contains over 1000 tU, with annual arisings of 190 tU. (Recovery of this by acid leaching is about 70%.)
A joint venture company, Yunnan Sparton New Environ-Tech Consulting Co (SNET) was set up to operate "the secondary uranium recovery programs in Yunnan", notably at Lincang, but no commercial recovery of uranium has been reported. Sparton also had an agreement to extract uranium from coal ash following germanium recovery in the Bangmai and Mengwang basins in Yunnan. This ash ranges from 150 to over 4000 ppm U (0.40% U), averaging 250 ppm U (0.025%). Sparton had an 85% interest in the Huajun germanium and coal mine, but does not mention uranium here. Sparton’s website at the end of 2014 has no mention of these projects.
In South Africa, HolGoun's Uranium and Power Project was investigating uranium recovery from the Springbok Flats coal field, estimated to contain 84,000 tU at grades of 0.06 to 0.10% U. The project is investigating the feasibility of mining the low-grade coal, using it to fire a conventional electricity generation plant, and extracting the uranium from the residual ash.
In Australia the NSW Aboriginal Lands Council has applied for a uranium exploration licence over four large coal ash dams adjacent to power stations.
Coal mining
Coal mining itself also gives rise to a potential NORM issue. Coal can be mined in either open pits or underground mines, and produces a significant amount of waste rock, and drainage water that can present with elevated levels of radioactivity. Underground coal mines are subject to increased radon levels, while elevated levels of radium and K-40 can be found in mining waste rocks and soil. Sediments discharged in waste water into the environment have been measured with activities as high as 55,000 Bq/kg of Ra-226 and 15,000 Bq/kg of Ra-228. (IAEA 2003, Tech Report 419)
A survey of 44 Chinese coal mines (40 of which were underground operations) indicated that radon concentrations in 15% of them were above 1000 Bq/m3. (NORM VII proceedings, IAEA 2015)
Oil and gas production
Analysis of oil and gas from many different wells has shown that the long-lived uranium and thorium isotopes are not mobilized from the rock formations that contain them. However Ra-226, Ra-224, Ra-228 and Pb-210 are mobilized, and appear mainly in the water co-produced during oil and gas extraction. These isotopes and their radioactive progeny can then precipitate out of solution, along with sulphate and carbonate deposits as scale or sludge in pipes and related equipment. Radon-222 is the immediate decay product of radium-226 and preferentially follows gas lines. It decays (through several rapid steps) to Pb-210 which can therefore build up as a thin film in gas extraction equipment.
The level of reported radioactivity varies significantly, depending on the radioactivity of the reservoir rock and the salinity of the water co-produced from the well. The higher the salinity the more NORM is likely to be mobilized. Since salinity often increase with the age of a well, old wells tend to exhibit higher NORM levels than younger ones. Table 4 gives the characteristics of NORM produced during oil and gas extraction and some indicative measurements of concentrations.
Table 4: NORM in oil and gas production
| Radionuclide | Natural gas Bq/m3 | Produced water Bq/L | Hard scale Bq/kg | Sludge Bq/kg |
|---|---|---|---|---|
| U-238 | trace | 1 - 500 | 5 - 10 | |
| Ra-226 | 0.002 - 1200 | 100 - 15 million | 50 - 800,000 | |
| Po-210 | 0.002 - 0.08 | 20 - 1500 | 4 - 160,000 | |
| Pb-210 | 0.005 - 0.02 | 0.05 - 190 | 20 - 75,000 | 10 - 1.3 million |
| Rn-222 | 5 - 200,000 | |||
| Th-232 | trace | 1 - 2 | 2 - 10 | |
| Ra-228 | 0.3 - 180 | 50 - 2.8 million | 500 - 50,000 | |
| Ra-224 | 0.05 - 40 |
Source: IAEA 2003, Safety Report Series 34.
If the scale has an activity of 30,000 Bq/kg it is 'contaminated', according to Victorian regulations. This means that for Ra-226 scale (decay series of nine progeny) the level of Ra-226 itself is 3300 Bq/kg. For Pb-210 scale (decay series of three) the level is 10,000 Bq/kg. These figures refer to the scale, not the overall mass of pipes or other material (cf Recycling section below). A 2010 analytical report shows Pb-210 scale at 18.6 MBq/kg from a pipeline in Canada.
For seawater injection systems a further NORM issue has more recently come to light: that of bio-film deposits fixing significant amounts of the seawater’s uranium.
Fracking (hydraulic fracturing) for gas production releases significant NORM in some geological environments, both in drill cuttings and water. In the US Marcellus shale in Pennsylvania, New York and West Virginia (a black shale) typically activity is about 370 Bq/kg including high levels of radium-226, giving up to 625 Bq/L in brine and up to 66 Bq/L in other water returned to the surface. US Geological Survey figures for brine are reported as 377 Bq/L Ra-226 and 46 Bq/L for Ra-228. Other reports related wastewater here to the drinking water standard (0.185 Bq/L) and said it was 300 times Nuclear Regulatory Commission limits for industrial wastewater discharge.
NORM in the oil and gas industry poses a problem to workers particularly during maintenance, waste transport and processing, and decommissioning. In particular Pb-210 deposits and films, as a beta emitter, is only a concern when pipe internals become exposed. External exposure due to NORM in the oil and gas industry are generally low enough not to require protective measures to ensure that workers stay beneath their annual dose limits (such as set out by the IAEA basic safety standards). Internal exposures can be minimized by hygiene practices.
Metals and smelting
The mining and processing of metal ores, other than uranium, may also generate large quantities of NORM wastes. These wastes include ore tailings and smelter slag, some of which contain elevated concentrations of uranium, thorium, radium and their decay products that were originally part of the process feed ore. As with coal, the level of NORM encountered varies by region and geological formation. Typically the radioactivity in the wastes may reach in the order of thousands of bequerels per kilogram, e.g. 3500 Bq/kg U-238 and 8800 Bq/kg Pb-210 in South African copper tailings. Only special use metals and the rare earth metals go beyond this. These are discussed below.
Radon exposure is often an issue in metal mines, and a survey of 25 underground mines in China showed six having radon concentrations of over the control limit of 1000 Bq/m3. In all the metal mines the annual average effective dose from radon and radon progeny was 7.75 mSv.
Mineral sands
Mineral sands contain zircon, ilmenite, and rutile, with xenotime and monazite. These minerals are mined in many countries and production amounts to millions of tonnes per year of zirconium and titanium (from rutile and ilmenite), though thorium, tin and the rare earth elements are associated. The NORM aspect is due to monazite – a rare earth phosphate containing a variety of rare earth minerals (particularly cerium and lanthanum) and 5-12% (typically about 7%) thorium, and xenotime – yttrium phosphate with traces of uranium and thorium.
The minerals in the sands are subject to gravity concentration, and some concentrates are significantly radioactive, up to 4000 Bq/kg. Most of this NORM ends up in the waste streams from mineral processing (often including monazite) and so, apart from zircon, the final product is itself devoid of NORM. However, sometimes niobium and tantalum are recovered from the waste stream, and residues may be used as either landfill or in construction sites where there is a possibility of public exposure.
Table 5: Radioactivity in mineral sands and products
| Thorium | Uranium | |||
|---|---|---|---|---|
| ppm | Bq/kg | ppm | Bq/kg | |
| Ore | 5-70 | 40-600 | 3-10 | 70-250 |
| Heavy mineral concentrate | 80-800 | 600-6600 | <10-70 | <250-1700 |
| Ilmenite | 50-500 | 400-4100 | <10-30 | <250-750 |
| Rutile | <50-350 | <400-2900 | <10-20 | <250-500 |
| Zircon | 150-300 | 1200-2500 | 150-300 | 3700-7400 |
| Monazite concentrate | 10,000-55,000 | 80,000-450,000 | 500-2500 | 12,000-60,000 |
| Processing tailings (incl monazite) | 200-6000 | 1500-50,000 | 10-1000 | 250-25,000 |
IAEA Technical Reports Series no. 419, p 84. NORM VII reported 29,000 Bq/kg Th-232 for zircon in Nigeria
See also Appendix: Mineral Sands
Over 95% of the market for zirconium requires it in the form of zircon (zirconium silicate). This mineral occurs naturally and is mined, requiring little processing. It is used chiefly in foundries, refractories manufacture and the ceramics industry. Zircons typically have activities of up to 10,000 Bg/kg of U-238 and Th-232. No attempt is usually made to remove radionuclides from the zircon as this is not economical. Because zircon is used directly in the manufacture of refractory materials and glazes, the products will contain similar amounts of radioactivity. Higher concentrations may be found in zirconia (zirconium oxide), which is produced by high temperature fusion of zircon to separate the silica. Zirconium metal manufacture involves a chlorination process to convert the oxide to zirconium chloride, which is then reduced to the metal.
During mining and milling of zircon, care must be taken to keep dust levels down. Then when zircon is fused in refractories or ceramics manufacture, silica dust and fumes must be collected. This may contain the more volatile radionuclides, Pb-210 and Po-210, and the collection of these gases means that pipeworks and filters become contaminated. The main radiological issue is occupational exposure to these radionuclides in airborne dusts in the processing plant. Waste produced during zirconia/zirconium production can be high in Ra-226, which presents a gamma hazard, and waste must be stored in metal containers in special repositories. Powders from filters used during zirconia manufacture have been assayed as high as 200,000Bq/kg of Pb-210 and 600,000 Bq/kg Po-210.
Tin production
Tin is sometimes a by-product of mineral sand production. Slag from smelting tin often contain high levels of niobium and tantalum and so may form the feedstock for their extraction. It also typically contains enhanced level of radionuclides.
Tantulum and Niobium
Tantalum usually occurs with the chemically-similar niobium, often in tantalite and columbite, coltan (columbite + tanatalite), or polychlore (niobium). Tantalum ores, often derived from pegmatites, comprise a wide variety of more than a hundred minerals, some of which contain uranium and/or thorium. Hence the mined ore and concentrate contain both these and their decay products in their crystal lattice. Concentration of the tantalum minerals is generally by gravity methods (as with mineral sands), so the lattice-bound radioisotope impurities if present will report with the concentrate.
While this has little radiological significance in the processing plant, tantalum concentrates shipped to customers sometimes exceed the Transport Code threshold of 10 kBq/kg, requiring declaration and some special documentation, labeling and handling procedures. Some reaches 75 kBq/kg.
Niobium slags can reach radioactivity levels in excess of 100 kBq/kg. Average activity concentrations associated with columbite-tantalite (coltan) small-scale artisanal mining and processing activities undertaken by hand in Rwanda are 600 Bq/kg for the ore and of the order of 1000–2000 Bq/kg for processed material. (NORM VII)
The largest producers of tantalum are Australia and Africa, most niobium comes from Brazil.
Rare Earth Elements
Rare Earth Elements (REEs) are chemically rather similar to uranium and thorium they are often found in conjunction with these radionuclides.
Rare earth elements (REE) are a set of seventeen chemical elements in the periodic table, specifically the fifteen contiguous lanthanoids plus the lighter scandium and yttrium. Scandium and yttrium are considered REE since they tend to occur in the same ore deposits as the lanthanoids and exhibit similar chemical properties. Most REEs are not rare. However, because of their geochemical properties, REE minerals are typically dispersed and not often found in concentrated and economically exploitable forms. REEs are often found together, and are difficult to separate. Many contain thorium, and some are associated with uranium. Monazite includes cerium as well as thorium, and associated light REEs, xenotime incorporates yttrium and heavy REEs.
The production of REEs has been accompanied by the production of large volumes of thorium hydroxide and residues containing radioactive lead and radium. In China, 30,000 tonnes of NORM residues are in temporary storage. Monazites form in phosphatic pegmatites and so REE extraction is sometimes in conjunction with phosphate mining.
In the Lincang coal deposit southwest of Kunming in China the lignite is enriched in uranium (100 to 4960 Bq/kg, average 1200), but not thorium or potassium. The coal is burned in blast furnaces and its fly ash removed from the bag filters is a source of rare earth concentrates, at 2.32% compared with 0.053% in the original coal. Radionuclides (apart from Pb & Po) are mostly in the bottom ash but also the flyash. About 1% of flyash and a lot of the volatile radionuclides are released to the atmosphere. In 2010, the activity in the coal was about 58 GBq for each radionuclide in uranium decay series, and that as volatiles released to the atmosphere from the plant was 15.5 GBq for U-238 (26% of original in coal), 11.7 GBq for Ra-226 (21%), 41.4 GBq for Pb-210 (71%) and 50.7 GBq for Po-210 (89%), plus a very small amount in the flyash. The release of radionuclides in the off-gas was much greater than the amount contained in the fly ash. (Wu et al in NORM VII)
See also paper: Uranium from Rare Earth Deposits
Uranium production
Though not normally considered as NORM, wastes from the front end of the nuclear fuel cycle through to fuel fabrication may be treated as NORM, opening up more options for disposal. Such material includes uranium oxides. Radon exposure is also an issue in uranium mines.
Phosphates and fertilizer production
Phosphate rock used for fertiliser is a major NORM due to both uranium and thorium. Phosphate is a common chemical constituent of fertilizer. It is principally mined from apatite and phosphate rocks (phosphorite) in which the concentration of phosphate has been enhanced by sedimentary, igneous, weathering and biological processes. Uranium can also be concentrated in these processes so that a high phosphate content generally coincide with high uranium content (50 -300ppm). Thorium is more likely to be present in igneous phosphorite. The radioactivity of these ores (due to uranium, thorium and radium) can be as high as 10,000 Bq/kg. Significant phosphate mining operations take place in many countries, with large outputs from the USA, Morocco and China, the world total being 156 Mt in 2007.
Table 6: Concentration of NORM radionuclides in phosphate rocks
| Country | Uranium (Bq/kg) | Thorium (Bq/kg) | Ra-226 (Bq/kg) | Ra-228 (Bq/kg) |
|---|---|---|---|---|
| USA | 259-3700 | 3.7-22 | 1540 | |
| USA: Florida | 1500-1900 | 16-59 | 1800 | |
| Brazil | 114-880 | 204-753 | 330-700 | 350-1550 |
| Chile | 40 | 30 | 40 | |
| Algeria | 1295 | 56 | 1150 | |
| Morocco | 1500-1700 | 10-200 | 1500-1700 | |
| Senegal | 1332 | 67 | 1370 | |
| Tunisia | 590 | 92 | 520 | |
| Egypt | 1520 | 26 | 1370 | |
| Jordan | 1300-1850 | |||
| Australia | 15-900 | 5-47 | 28-90 |
source: IAEA Technical Reports Series no. 419, p90
Phosphoric acid is an intermediate step in almost all phosphate applications. Production requires first the beneficiation of the ore, followed by acid leaching and separation. In general the beneficiation stage does not result in a reduction of NORM in the ore.
Treatment with sulfuric acid leads to the production of gypsum (phosphogypsum) which retains about 80% of Ra-226 and 30% of Th-232 and 14% of U-238. This means that uranium and thorium are enhanced to about 150% of the value of the beneficiated ore, making it a significant NORM. This gypsum can either be sold or disposed of. In the USA, the use of phosphogypsum with a radioactivity greater than 370 Bq/kg is banned by the Environmental Protection Authority. Gypsum can either be disposed of in piles or discharged to rivers and the sea. Some leaching from the material is possible. Gypsum wastes can have radioactivity levels up to 1700 Bq/kg. Scales from the sulfuric acid process are formed in the pipes and filtration systems of plants and need to be cleaned or replaced periodically. While much smaller in volume than gypsum, these wastes can be much more radioactive – even over 1MBq/kg.
Processing phosphate sometimes gives rise to measurable doses of radiation to people. Phosphate rocks containing up to 120 ppm U have been used as a source of uranium as byproduct – some 17,000 tU in the USA, and are likely to be so again.
See also the paper Uranium from Phosphate Deposits.
Table 7: Concentration of radionuclides in fertilisers (Bq/kg)
| Products | U-238 | Ra-226 | Th-232 |
|---|---|---|---|
| Phosphoric acid | 1200-1500 | 300 | - |
| Normal super-phosphate | 520-1100 | 110-960 | 15-44 |
| Triple superphosphate | 800-2160 | 230-800 | 44-48 |
| Mono-ammonium phosphate | 2000 | 20 | 63 |
| Diammonium phosphate | 2300 | 210 | <15 |
| Dicalcium phosphate | - | 740 | <37 |
| PK fertilizer | 410 | 370 | <15 |
| NP fertiliser | 920 | 310 | <30 |
| NPK fertiliser | 440-470 | 210-270 | <15 |
Source: IAEA Technical Reports Series no. 419, p100
European fertiliser manufacturing gave rise to discharges of phosphogypsum containing about 4 TBq/yr of Ra-226, Pb-210 and Po-210 into the North Sea and North Atlantic. This reduced to about half the amount in the 1990s, and was overtaken as a source of radioactivity by offshore oil and gas production in Norwegian and UK waters, releasing over 10 TBq/yr of Ra-226, Ra-228 & Pb-210. This means that together they contribute 95% of the alpha-active discharges in those waters (two orders of magnitude more than the nuclear industry, and with this NORM having higher radiotoxicity).
Building Materials
Building materials can contain elevated levels of radionuclides including particularly Ra-226, Th-232 and K-40, these three being collectively the basis of the activity concentration index (ACI) approach adopted throughout Europe. K-40 is most significant in published Australian data, ranging up to 4000 Bq/kg in natural stone and 1600 Bq/kg in clay bricks and concrete. Bricks can also contain up to 2200 Bq/kg of Ra-226 (Cooper 2005).
Activity concentration guidelines for the use of NORM residues in building construction have been developed using the ACI approach and material has been classified into three categories, depending on whether the dose is below 0.5 mSv/yr (unrestricted use), between 0.5 and 1 mSv/yr (use restricted to roads, bridges, dams or, with dilution, low occupancy buildings) or above 1 mSv/yr (prohibited use). These levels correspond to equivalent activity concentration under 350 Bq/kg (and under 200 Bq/kg Ra-226), 350 to 1350 Bq/kg (200-1000 Bq/kg Ra-226) and over 1350 Bq/kg (1000 for Ra-226) respectively.
Granite, widely used as a cladding on city buildings and also architecturally in homes, contains an average of 3 ppm (40 Bq/kg) uranium and 17 ppm (70 Bq/kg) thorium. Radiation measurements on granite surfaces can show levels similar to those from low-grade uranium mine tailings. Table 8 shows some recorded activity concentrations for building materials. However some extreme values in excess of these have also been recorded.
Table 8: Activity concentrations of NORM in building materials (Bq/kg)
| Material | Ra-226 | Th-232 | K-40 |
|---|---|---|---|
| Concrete | 1-250 | 1-190 | 5-1570 |
| Aerated concrete | 109818 | <1-220 | 180-1600 |
| Clay bricks | 1-200 | 1-200 | 60-2000 |
| Sand-lime bricks and sandstone | 18415 | 10959 | 5-700 |
| Natural building stones | 1-500 | 1-310 | 767011 |
| Natural gypsum | <1-70 | <1-100 | 7-280 |
| Cement | 7-180 | 7-240 | 24-850 |
| Tiles | 30-200 | 20-200 | 160-1410 |
| Phosphogypsum | 4-700 | 19360 | 25-120 |
| Blast furnace slag stone and cement | 30-120 | 30-220 | - |
source: IAEA Technical Reports Series no. 419, p 104
The EU encourages the use of NORM residues in building materials, subject to dose rate from gamma exposure being below 1 mSv/yr from them. Coal ash and smelting slag are an important constituent of building materials in China.
Recycling and NORM
In 2015 the IAEA (NORM VII) says that there is still a lack of harmonization of national approaches to the management of NORM residues. However, acceptance of the need to minimize NORM waste by recycling NORM residues or using them as by-products (with dilution if necessary) continues to grow. Some national authorities are now actively promoting this approach instead of discouraging or prohibiting it as in the past. This includes use in building materials, subject to 1 mSv/yr reference level for exposure.
Earlier IAEA recommendations for the classification of exempt waste (i.e. beneath low-level, and therefore not requiring any special facilities for disposal) are between 10 Bq/g and 1 MBq/g for 'moderate amounts' – depending on the radionuclide in question and the chances of public exposure (Radiation Protection and Safety of Radiation Sources: International Basic Safety Standards, IAEA July 2014), however in practice categorization of waste is strongly determined by where the waste comes from.
For example, scrap steel from gas plants may be recycled if it has less than 500,000 Bq/kg (0.5 MBq/kg) radioactivity (the exemption level). This level however is one thousand times higher than the clearance level for recycled material (both steel and concrete) from the nuclear industry! Anything above 500 Bq/kg may not be cleared from regulatory control for recycling. Current IAEA Basic Safety Standards (BSS) clearance levels specify 1 Bq/g for natural radionuclides in the U-238 series in secular equilibrium with progeny, and the same for those in the Th-232 series. IAEA BSS clearance levels for bulk amounts being recycled are: Fe-55 1 MBq/kg, Co-60m 1 MBq/kg, Ni-63 100 kBq/kg, C-14 1 kBq/kg, Cs-137 0.1 kBq/kg, Ra-226 1 kBq/kg.
Decommissioning experts are increasingly concerned about double standards developing in Europe which allow 30 times the dose rate from non-nuclear recycled materials than from those out of the nuclear industry. In respect to actual dose limits, 0.3 to 1.0 mSv/yr individual dose constraint is applied to oil and gas recyclables, and 0.01 mSv/yr for release of materials with the same kind of radiation from the nuclear industry.
The concern arises because of the very large amounts of NORM needing recycling or disposal from many sources. The largest NORM waste stream is coal ash, with 280 million tonnes arising globally each year, and carrying U-238 and all its non-gaseous decay products, as well as Th-232 and its progeny. This is usually just buried. However, the double standard means that the same radionuclide, at the same concentration, can either be sent to deep disposal or released for use in building materials, depending on where it comes from. The 0.3 mSv/yr dose limit is still only one tenth of most natural background levels, and two orders of magnitude lower than those experienced naturally by many people, who suffer no apparent ill effects.
The main radionuclide in scrap from the oil and gas industry is radium-226, with a half-life of 1600 years as it decays to radon. Those in nuclear industry scrap are cobalt-60 and caesium-137, with much shorter half-lives. Application of a 0.3 mSv/yr dose limit results in a clearance level for Ra-226 of 500 Bq/kg for oil/gas scrap, compared with 10 Bq/kg for nuclear material.
In 2011, 16 decommissioned steam generators from Bruce Power in Canada were to be shipped to Sweden for recycling. Although the Canadian Nuclear Safety Commission (CNSC) approved Bruce Power’s plans in 2011 and confirmed steam generator processing is an excellent example of responsible and safe nuclear waste management practices, this caused public controversy at the time, and following the Fukushima nuclear accident plans for this shipment were shelved. These steam generators were each 12m long and 2.5m diameter, with mass 100 t, and contained some 4g of radionuclides with about 340 GBq of activity. Exposure was 0.08 mSv/hr at one metre. They were classified as low-level waste (LLW). Studsvik in Sweden would recycle much of the metal and return about 10% of the overall volume as LLW for disposal in Ontario. The balance would be under 100 Bq/kg, which appeared to be the clearance level.
Remediation of legacy sites
Typically a soil cleanup level of 0.5 to 1 Bq/g is a goal, though for residential land in UK 0.1 Bq/g is the level required. Material above the target level is sent to landfill, and anything over 100 Bq/g needs to be buried. Heavy metals may be of more concern than radionuclides in such situations. Following the Fukushima accident large areas were contaminated mainly with caesium fallout. In 2016 the government announced that material with less than 8 Bq/g caesium would no longer be subject to restriction regarding disposal.
Radon
Radium-226 is one of the decay products of uranium-238, which is widespread in most rocks and soils. When this radium decays it produces radon-222, an inert gas with a half-life of almost 4 days. (Radium-224 is a decay product of thorium, and it decays to radon-220, also known as thoron, with a 54-second half-life.) Because radon is so short-lived, and alpha-decays to a number of daughter products which are solid and very short-lived, there is a high probability of its decay when breathed in, or when radon daughter products in dust are breathed in. Alpha particles in the lung are hazardous.
Typically exposure to radon and its progeny accounts for half of an individual’s radiation dose, making it the single largest contributor. This radon comes from the ground, with exposure affected by factors such as local geography, building construction, and lifestyle. Radon levels in the air range from about 4 to 20 Bq/m3. Indoor radon levels have attracted a lot of interest since the 1970s and in USA they average about 55 Bq/m 3, with an EPA action level of 150 Bq/m3. Levels in Scandinavian homes are about double the US average, and those in Australian homes average one fifth of those in USA. Levels up to 100,000 Bq/m3 have been measured in US homes. In caves open to the public, levels of up to 25,000 Bq/m3 have been measured. A Japanese study on 3000 residents living in an area with 60 Bq/m3 radon near Misasa hot springs showed no health difference. The ICRP recommends keeping workplace radon levels below 300 Bq/m3, equivalent to about 10 mSv/yr.
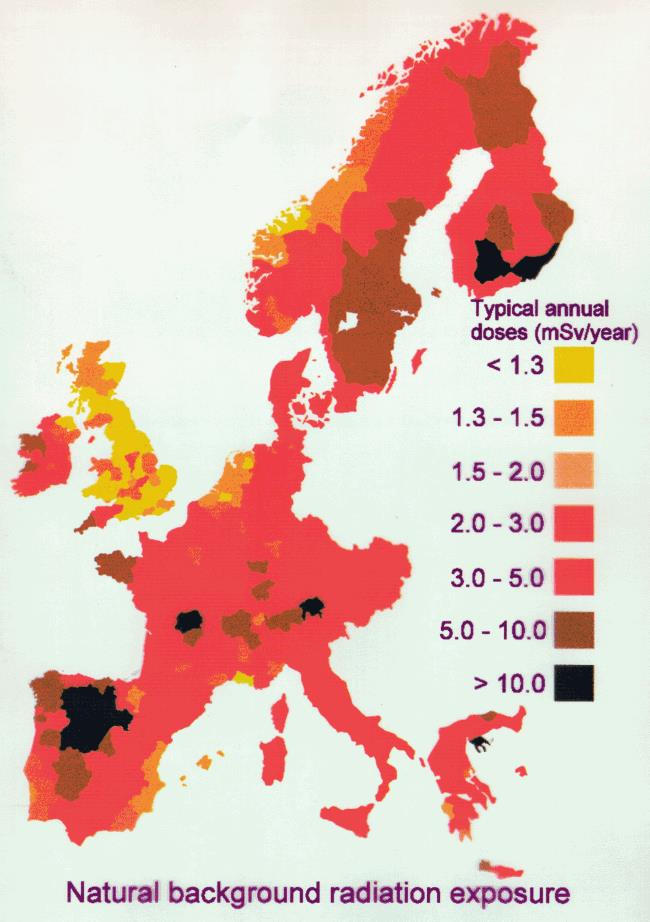
Figure 1 shows a map of some of the background radiation levels measured across parts of Europe. Much of this is due to the radon.
Figure 1: Natural background radiation in parts of Europe (source: Gonzalez 2011)
Radon also occurs in natural gas at up to 37,000 Bq/m3, but by the time it gets to consumers the radon has largely decayed. However, the solid decay products then contaminate gas processing plants, and this manifestation of NORM is an occupational health issue, as discussed above.
Exposure to radon is a problem in certain mining activities, notably uranium mining, and good ventilation must be assured so as to keep occupational exposure down, and levels must be monitored.
Sources:
Australian Nuclear Forum Inc., Information Paper No. 1, August 2002,Trace Elements in Australian Coals,
Argonne National Laboratory, Web page on the Naturally Occurring Radioactive Materials (NORM) program on the website for Environmental Science Division (www.evs.anl.gov), last accessed July 2011
Australian Radiation Protection and Nuclear Safety Agency's (Arpansa's) Radiation Health and Safety Advisory Council web page on Naturally Occurring Radioactive Material, last accessed July 2011.
Brookhaven National Laboratory, National Nuclear Data Centre website http://www.nndc.bnl.gov/ , accessed July 2011.
Cooper, M. B. 2005 Naturally Occurring Radioactive Materials (NORM) in Australian Industries - Review of Current Inventories and Future Generation, ERS-006, A Report prepared for the Radiation Health and Safety Advisory Council
Commonwealth Scientific and Industrial Research Organisation (CSIRO) website (www.csiro.au), Trace elements in Australian export thermal coals. Figures for average concentrations of uranium and thorium in Australian coal are in Fact Sheets on Uranium in Australian export thermal coals and Thorium in Australian export thermal coals
Dale, L., Trace Elements in Coal, Australian Coal Association Research Program (ACARP), Report No. 2 (October 2006)
Eisenbud, M.; and Gesell, T. F. 1997, Environmental Radioactivity from Natural, Industrial & Military Sources, Fourth Edition: From Natural, Industrial and Military Sources, Academic Press (ISBN: 9780122351549)
European Commission (Directorate-General Environment, Radiation Protection) 2003, Radiation protection 132: MARINA II, Update of the MARINA Project on the radiological exposure of the European Community from radioactivity in North European marine waters
European Commission (Directorate-General for Energy and Transport), 2003 Radiation Protection 135: Effluent and dose control from European Union NORM industries: Assessment of current situation and proposal for a harmonised Community approach, Volume 1: Main Report.
European Union Council Directive 2013/59/Euratom, http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2014:013:0001:0073:EN:PDF
Gabbard, A. 1993, Coal Combustion: Nuclear Resource or Danger?, Oak Ridge National Laboratory Review, Vol. 26, Nos. 3&4
Gooding, T.D.; Smith, K. R.; Sear, L.K. 2006, A radiological study of pulverised fuel ash (PFA) from UK coal-fired power stations, joint paper by the Health Protection Agency and the United Kingdom Quality Ash Association (UKQAA) presented at the UKQAA's Ash Technology Conference 2006 (AshTech 2006) held in Birmingham, UK on 15-17 May 2006
Gonzalez, A, J., 2011, Radiation Protection, presentation given at the World Nuclear University Event – ‘Key Issues in the World Nuclear Industry Today’, Ulaanbaatar, Mongolia.
International Atomic Energy Agency, 2014, Radiation Protection and Safety of Radiation Sources: International Basic Safety Standards, STI/PUB/1578 (July 2014)
International Atomic Energy Agency, Naturally Occurring Radioactive Material (NORM VII): Proceedings of an International Symposium Beijing, China, 22-26 April 2013, STI/PUB/1664, ISBN 9789201040145 (January 2015)
International Atomic Energy Agency, Extent of Environmental Contamination by Naturally Occurring Radioactive Material (NORM) and Technological Options for Mitigation, Technical Reports Series No. 419, STI/DOC/010/419, ISBN: 9201125038 (December 2003)
International Atomic Energy Agency, 2003, Radiation Protection and the Management of
Radioactive Waste in the Oil and Gas Industry, Safety Report Series No. 419, STI/PUB/1171 (ISBN: 9201140037)
McBride et al., 1977, Radiological Impact of Airborne Effluents of Coal-Fired and Nuclear Power Plants, Oak Ridge National Laboratory, ORNL-5315
Mishra, U. C. 2004, Journal of Environmental Radioactivity, Volume 72, Issues 1-2, Pages 35-40, Environmental impact of coal industry and thermal power plants in India.
Sparton Resources web page on uranium secondary recovery on the Sparton Resources website (www.spartonres.ca)
Swaine, D. J. Trace Elements in Coal, Butterworth-Heinemann, July 1990 (ISBN: 9780408033091)
United Kingdom Quality Ash Association (UKQAA) website www.ukqaa.org.uk. See also UKQAA Technical Datasheet 8.5, Radiation and Fly Ash
United Nations Scientific Committee on the Effects of Atomic Radiation, 2008, Exposures of the Public and Workers from Various Sources of Radiation, Annex B to Volume I Report to the General Assembly, Sources and Effects of Ionizing Radiation, available on the UNSCEAR 2008 Report Vol. I webpage
United Nations Scientific Committee on the Effects of Atomic Radiation, 2006, Sources-to-effects assessment for radon in homes and workplaces, Annex E to Volume II of the Report to the General Assembly, Effects of Ionizing Radiation, available on the UNSCEAR 2006 Report Vol. II webpage
United Nations Scientific Committee on the Effects of Atomic Radiation, 2000 Exposures from natural radiation sources, Annex B to Volume I of the Report to the General Assembly, Sources and Effects of Ionizing Radiation, available on the UNSCEAR 2000 Report Vol. I webpage (www.unscear.org/unscear/en/publications/2000_1.html)
U.S. Energy Information Administration (April 2010) U.S. Coal Supply and Demand 2009 Review.
U.S. Geological Survey, Fact Sheet FS-163-97, 1997 Radioactive Elements in Coal and Fly Ash: Abundance, Forms, and Environmental Significance.