The Cosmic Origins of Uranium
- Uranium is used to generate about 9% of our electricity worldwide, yet this fact pales into insignificance when we consider the role uranium has played in the evolution of the Earth.
- The Earth's uranium had been thought to be produced in one or more supernovae over 6 billion years ago. More recent research suggests some uranium is formed in the merger of neutron stars.
- Uranium later became enriched in the continental crust.
- Radioactive decay contributes about half of the Earth’s heat flux.
Geologists and geochemists have been studying the abundance, distribution and chronometric potential of the isotopes of uranium for more than a century. Their work stems from Klaproth's discovery in 1789 of the heaviest naturally occurring element, Becquerel's demonstration in 1896 that uranium salts are radioactive, Boltwood's conclusion in 1905 that lead as well as helium is a decay product of uranium, and Rutherford's suggestion in 1906 of the geological time-keeping potential of radioactivity. From a geochemical point of view, some of the major questions are:
- Where did the uranium now in the Earth come from?
- What effects has the comparatively trivial uranium content of the Earth had on the evolution of the planet and, conversely, are there feedbacks controlling the geochemical cycle of uranium that vary secularly (i.e. over long, indefinite periods of time)?
- Can we track through time the way uranium has been recycled through the exosphere, crust and mantle of the Earth?
Cosmic abundance of elements
For many years, since the 1930s, a large number of scientists have been occupied with determining the abundances of the elements and their isotopes in the objects comprising the solar system, and with accounting for the abundance patterns observed. In fact, spectroscopic measurements show that the abundances of elements in stars vary and that there is no single applicable 'cosmic abundance' pattern.
Closer to home, there are major differences in abundances of the elements in the various planets that orbit our hydrogen-helium dominated Sun. The terrestrial planets, including the Earth, are relatively depleted in the potentially gaseous or volatile elements (hydrogen, helium, carbon and neon) and are dominated by elements of low and even atomic number (oxygen, magnesium, silicon and iron). On this scale, uranium – the abundance of which in the Sun is only 10-12 that of hydrogen – is an exceedingly rare element. Furthermore, measurements of oxygen isotopes in meteorites show that the solar system as a whole is not homogeneous in terms of isotopic ratios. All these variations point to a conclusion that multiple sources were involved in the production of proto-solar system material.
Where did uranium come from?
Cosmochemists have been concerned not only with patterns and secular trends of abundance of the elements in galaxies but also with the origins of abundance anomalies in particular stars and with theories on the synthesis of different nuclei to account for these observations. According to these theories, the Earth's uranium was produced in one or more supernovae ("An explosive brightening of a star in which the energy radiated by it increases by a factor of ten billion... A supernova explosion occurs when a star has burned up all its available nuclear fuel and the core collapses catastrophically." - Oxford Dictionary of Physics). The main process concerned was the rapid capture of neutrons on seed nuclei at rates greater than disintegration through radioactivity. The neutron fluxes required are believed to occur during the catastrophically explosive stellar events called supernovae. Gravitational compression of iron (the island of nuclear stability, incapable of further exothermic fusion reactions) and sudden collapse in the centre of a massive star triggers the explosive ejection of much of the star into space, together with a flood of neutrons. Remnants of hundreds of supernovae have been found.
More recently, a second theory has proposed that uranium is created during the merger of two neutron stars. Neutron stars are very dense, with a teaspoon of neutron star material having a mass of the order of 5 billion tonnes. When two such bodies come close together the intense gravitational forces cause them to violently merge, giving off gravitational waves and producing huge amounts of heavy elements, such as gold, platinum and uranium.
So, we know that the Earth's uranium was produced through one or more of these processes, and that this material was inherited by the solar system of which the Earth is a part.
We can estimate how long ago this synthesis of uranium occurred, given:
- The present day abundances of U-235 and U-238 in the various 'shells' forming our planet.
- A knowledge of the half-lives of these isotopes.
- The age of the Earth (ca. 4.55 billion years) – known from various radiometric 'clocks', including those of the uranium-to-lead decay chains.
We can calculate the abundances of U-235 and U-238 at the time the Earth was formed. Knowing further that the production ratio of U-235 to U-238 in a supernova is about 1.65, we can calculate that if all of the uranium now in the solar system were made in a single supernova, this event must have occurred some 6.5 billion years ago.
This 'single stage' is, however, an oversimplification. In fact, multiple supernovae from over 6 billion to about 200 million years ago were involved. Additionally, studies of the isotopic abundances of elements, such as silicon and carbon in meteorites, have shown that more than ten separate stellar sources were involved in the genesis of solar system material. Thus the relative abundance of U-235 and U-238 at the time of formation of the solar system:
- Cannot be inverted to a 'single stage' model age.
- Is essentially an accidental and unique value.
- Reflects the input of the explosive debris of many progenitor stars.
Enrichment in Earth's crust
Many analyses have been made of the uranium in the rocks forming the continental and oceanic crusts, and in samples of the Earth's mantle exposed as uplifted slices in mountain belts or as 'xenoliths' in basalts and kimberlites (hosts of diamonds).
We can have some confidence that these measurements are robust for the crust and upper mantle of the Earth, but less confidence that we know the abundance of uranium in the lower mantle and the outer and inner cores. While on average the abundance of uranium in meteorites is about 0.008 parts per million (gram/tonne), the abundance of uranium in the Earth's 'primitive mantle' – prior to the extraction of the continental crust – is 0.021 ppm. Allowing for the extraction of a core-forming iron-nickel alloy with no uranium (because of the characteristic of uranium which makes it combine more readily with minerals in crustal rocks rather than iron-rich ones), this still represents a roughly twofold enrichment in the materials forming the proto-Earth compared with average meteoritic materials.
The present-day abundance of uranium in the 'depleted' mantle exposed on the ocean floor is about 0.004 ppm. The continental crust, on the other hand, is relatively enriched in uranium at some 1.4 ppm. This represents a 70-fold enrichment compared with the primitive mantle. In fact, the uranium lost from the 'depleted' oceanic mantle is mostly sequestered in the continental crust.
It is likely that the process or processes which transferred uranium from the mantle to the continental crust are complex and multi-step. However, for at least the past 2 billion years they have involved:
- Formation of oceanic crust and lithosphere through melting of the mantle at mid-ocean ridges.
- Migration of this oceanic lithosphere laterally to a site of plate consumption (this is marked at the surface by a deep-sea trench).
- Production of fluids and magmas from the downgoing (subducted) lithospheric plate and overriding mantle 'wedge' in these subduction zones.
- Transfer of these fluids/melts to the surface in zones of 'island arcs' (such as the Pacific's Ring of Fire).
- Production of continental crust from these island arc protoliths, through remelting, granite formation and intra-crustal recycling.
Uranium enrichment in The Earth's crust
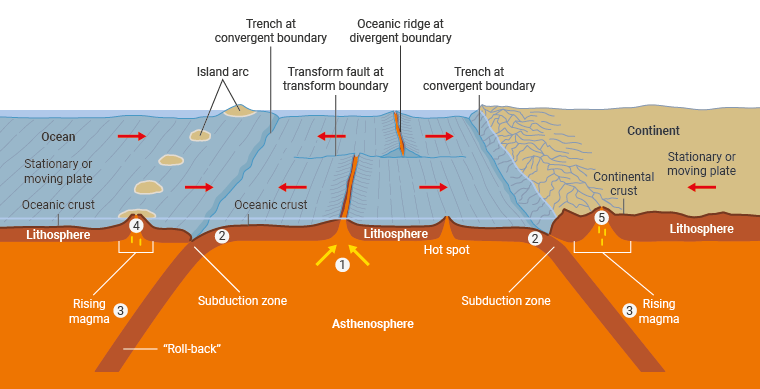
Source: adapted from Encyclopedia Britannica
All through this crust-forming cycle, the lithophile character of uranium is manifest in the constancy of the potassium to uranium ratio at about 10,000 in the rock range from peridotite to granite. Because we would like to keep track of how uranium is distributed in the Earth, the abundance and isotopic characteristics of lead – the radiogenic daughter of U-235 and U-238 – are useful parameters. Table 1 below highlights the relatively low abundance of lead in the Earth's mantle and the consequent high uranium to lead ratio, compared with meteorites. The difference in abundance can most likely be explained by lead's volatile nature and tendency to combine with iron, with lead being lost during terrestrial accretion and core separation. One of the consequences, of course, of these high ratios is the comparatively high radiogenic/non-radiogenic content of Pb-207/Pb-204 and Pb-206/Pb-204 in the Earth's crust and mantle compared with meteorites or the earth's core. (Pb-207 is the final stable decay product of U-235, and Pb-206 is that of U-238. Pb-204 is non-radiogenic.
Table 1
| U abundance (ppm) |
Pb abundance (ppm) |
U/Pb ratio | |
| Meteorites | 0.008 | 2.470 | 0.003 |
|---|---|---|---|
| Primitive mantle | 0.021 | 0.185 | 0.113 |
| Continental crust | 1.4 | 12.6 | 0.111 |
The figure given for the continental crust is an average of the entire crust. Of course, local concentration of uranium can far exceed these values, ranging up to 50 ppm disseminated in some granites, to much higher values in ore deposits. In fact, in the geological past, local concentrations of uranium have occasionally achieved natural criticality, for example the Oklo reactors in Gabon (see below).
Energy source
Convection in the outer core and the mantle, whereby heat is transferred by movement of heated matter, governs many of the Earth's endogenous processes.
The convection in the core may be driven by the heat released during progressive solidification of the core (latent heat of crystallization) and leads to the self-sustaining terrestrial dynamo which is the source of the Earth's magnetic field. Heat transfer from the core at the core/mantle boundary is also believed to trigger upwellings of relatively hot, and hence low density, plumes of material. These plumes then ascend, essentially without gaining or losing heat, and undergo decompression melting close to the Earth's surface at 'hot spots' like Hawaii, Reunion and Samoa.
However, the primary source of energy driving the convection in the mantle is the radioactive decay of uranium, thorium and potassium. In the present Earth, most of the energy generated is from the decay of U-238 (ca. 10-4 watt/kg). At the time of the Earth's formation, however, decay of both U-235 and K-40 would have been about equal in importance and both would have exceeded the heat production of U-238.
A simple way of viewing the process of plate tectonics – the formation and disposal of oceanic lithosphere – is that this is the mechanism by which the mantle sheds heat. Conversely, 'mantle plumes/hot spots' are the way the core sheds heat. In terms of total heat loss from the Earth at present, plate activity constitutes about 74%, hot spots account for approximately 9% and radiogenic heat lost directly from the continental crust is some 17%. The Earth is well insulated thermally and the heat loss from the surface now can reflect heat generation a considerable time in the past.
Measurements of heat have led to estimates that the Earth is generating between 30 and 44 terawatts of heat, much of it from radioactive decay. Measurements of antineutrinos have provisionally suggested that about 24 TW arises from radioactive decay. Professor Bob White provides the more recent figure of 17 TW from radioactive decay in the mantle, and a more recent figure based on geoneutrinos is 20 +/- 8 TW from U-238 and Th-232 decay, plus 4 TW from K-40. This compares with 42-44 TW heat loss at the Earth's surface from the deep Earth. The balance comes from changes in the core. Thus about half the Earth’s total heat flux is from radioactive decay. (There is very much greater heat loss arising from incident solar radiation, which is quite distinct.)
Atmosphere and greenhouse effect, the role of plants
Apart from the fundamental physical and chemical differentiation of the Earth driven by plate tectonics, lithosphere formation and destruction are also critical for many processes in the outer layer of the atmosphere. We know, for example, that during periods of enhanced oceanic lithosphere formation, such as occurred during the Cretaceous period some 100 million years ago, the mid-ocean ridges stood higher, triggering flooding of the low-lying portions of the continents. In fact the Laurasian part of the former Pangea supercontinent was drowned to a greater extent than the Gondwana part, maybe reflecting some deep-seated thermal/compositional contrast. The effects were many and include:
- Enhanced carbon dioxide release causing increased carbon dioxide content of the ocean and the atmosphere
- Diminished continental surface area leading to a reduction in the titration through weathering of atmospheric carbon dioxide
- Sustained high atmospheric carbon dioxide levels leading to an enhanced greenhouse effect and warmer climate.
Secular changes have taken place in several atmospheric processes, including a change in composition, from relatively reducing to astonishingly oxidising. The odd-looking "equation" for atmosphere production is:
CO2 + H2 = N2 + O2
where the primary, volcanically degassed inputs to the atmosphere are on the left, and the cumulative abundant components are on the right hand side of the equation. Nitrogen is a trace volcanic emission, not utilized to any great extent in surface processes – including the trivial effect of organic life – and merely accumulates in the atmosphere. The Earth's distance from the Sun, together with the greenhouse feedback, allows surface temperatures to be generally sustained within the condensation range for water. Carbon dioxide dissolves in water and is also sequestered in calcite by inorganic and organic precipitation as limestone.
The remarkable feature of our atmosphere is the presence of molecular oxygen released through photosynthesis, the process by which green plants manufacture their carbohydrates from atmospheric carbon dioxide and water:
6CO2 + 6H2O = C6H12O6 + 6O2
Photosynthesis can be traced back in time to about 3.8 billion years. For a while, the oxygen released was consumed through oxidation of reduced ferrous compounds at the Earth's surface. Ultimately, the oxygen started to accumulate in the atmosphere as free oxygen some 2.5 billion years ago.
In addition to many other effects, the change in the redox character of the exosphere led to a fundamental change in the way uranium was transported in the weathering-erosion-deposition cycle. Whereas under reduced conditions uranium is relatively insoluble and stable as uraninite (UO2), under oxidizing conditions it becomes soluble (U6+) and readily transported. Since 2.5 billion years ago, ore deposits of uranium have been formed primarily where reduction of uranium-bearing fluids was achieved, for example by bacteria or through contact with graphitic shales.
Uranium distribution through time
The oxidizing atmosphere also led to an increased concentration of uranium in the oceans. As a consequence, transport in recirculating hydrothermal fluids led to relative enrichment in the oceanic crust too. The enhanced uranium transport from the exosphere to the Earth's interior – via subduction of oceanic lithosphere and the subsequent rehomogenization of this lithosphere into the Earth's mantle – has had a significant effect on the present distribution of uranium in the Earth, and may account for some curious inconsistencies in the isotopic characteristics of the mantle. For example, whereas the time-integrated Pb-208 (stable final decay product of Th-232)/Pb-206 values of mid-ocean ridge basalts indicate mantle source values of Th/U of about 4, the measured values of Th/U and systematics of short-lived Th-U disequilibria indicate a ratio of about 2. It is likely that since about 2.5 billion years ago injections of uranium into the mantle have been effective in the reduction of the thorium to uranium ratio on an (upper) mantle-wide scale.
An additional net effect is the selective reinjection of uranium as opposed to lead – which is mostly stripped out in subduction zones and returned immediately to the crust – into the mantle. We know that overall, most basalts are being produced from a mantle with an enhanced uranium/lead ratio and with apparent 'future' ages, compared with the lead isotopic ratios characteristic of a closed system, single stage evolution of uranium/lead in the Earth. This feature is sometimes referred to by geochemists as the 'lead paradox', and may in part relate to the feedback influence of an oxidising, life-triggered exosphere on the interior of the Earth.
Natural nuclear reactors in the Earth's crust
At Oklo in Gabon, West Africa, about 2 billion years ago, at least 17 natural nuclear reactors commenced operation in a rich deposit of uranium ore. Each operated at about 20 kW thermal. At that time the concentration of U-235 in all natural uranium was 3.7 percent instead of 0.7 percent as at present (U-235 decays much faster than U-238, whose half-life is about the same as the age of this planet.).
These natural chain reactions, started spontaneously by the presence of water acting as a moderator, continued for about two million years before finally dying away. During this long reaction period about 5.4 tonnes of fission products as well as 1.5 tonnes of plutonium together with other transuranic elements were generated in the orebody. The initial radioactive products have long since decayed into stable elements but study of the amount and location of these has shown that there was little movement of radioactive wastes during and after the nuclear reactions. Plutonium and the other transuranics remained immobile.
Georeactor theory
A quite different view of the role of uranium in the Earth is the theory that much of the uranium in the primordial planet sunk to the core and has formed a core there, some 8 km across, which has been fissioning ever since. The depletion of U-235 over geological time has not terminated the reaction because this core is a fast reactor (not requiring any moderator) which breeds plutonium-239 from the U-238. The georeactor theory rests on relatively little evidence, and is not widely supported.
Notes & references
This page has been adapted from a paper presented by Professor Richard Arculus at the Uranium Institute Mid-Term Meeting in Adelaide on 17 April 1996. The paper was used with the author's permission.